We believe in clinical trials enriched with responders to treatment – accelerating approvals of life-changing therapies
Unlock the power of mechanistic biomarkers to maximize probability of trial success
We go beyond conventional biomarker discovery to deliver mechanistically driven clinical biomarkers that map drug candidates to the underlying biology of patients in the trial's primary indication.
Using our platform, we stratify patient populations to identify subgroups based on shared disease mechanisms. This enables biomarker-guided development strategies that enrich trials with responsive participants to eliminate risk and maximize the probability of success across all disease areas.
This is precision drug development.
2.6x greater
probability of trial success for drugs with genetic support.
We go much further.
Succeed where others would fail
Our unique understanding of in which patients a drug candidate will be effective is key to the success of clinical programs.
We identify the causal biology of disease and find biomarkers that reflect the mechanisms responsible for disease progression and treatment response in well-defined patient subgroups.
Patient stratification biomarkers to identify and recruit responsive subpopulations.
Predictive biomarkers to distinguish the biology underpinning responders from non-responders before or early in treatment.
Mechanistic biomarkers that align with drug MoA to support regulatory submissions and development of companion diagnostics.
Find and recruit super responders
We identify and enable enrollment of the most scientifically robust and commercially attractive patient populations.
In clinical development, we enable our partners to:
Stratify and understand the mechanisms driving disease
Discover biomarkers associated with drug response
Enhance statistical power and detect efficacy signals earlier
Develop complementary diagnostics to identify responders
Succeed with smaller trials that are faster to readout
Our biomarker-led development strategies de-risk clinical programs, improve regulatory confidence and maximize value.
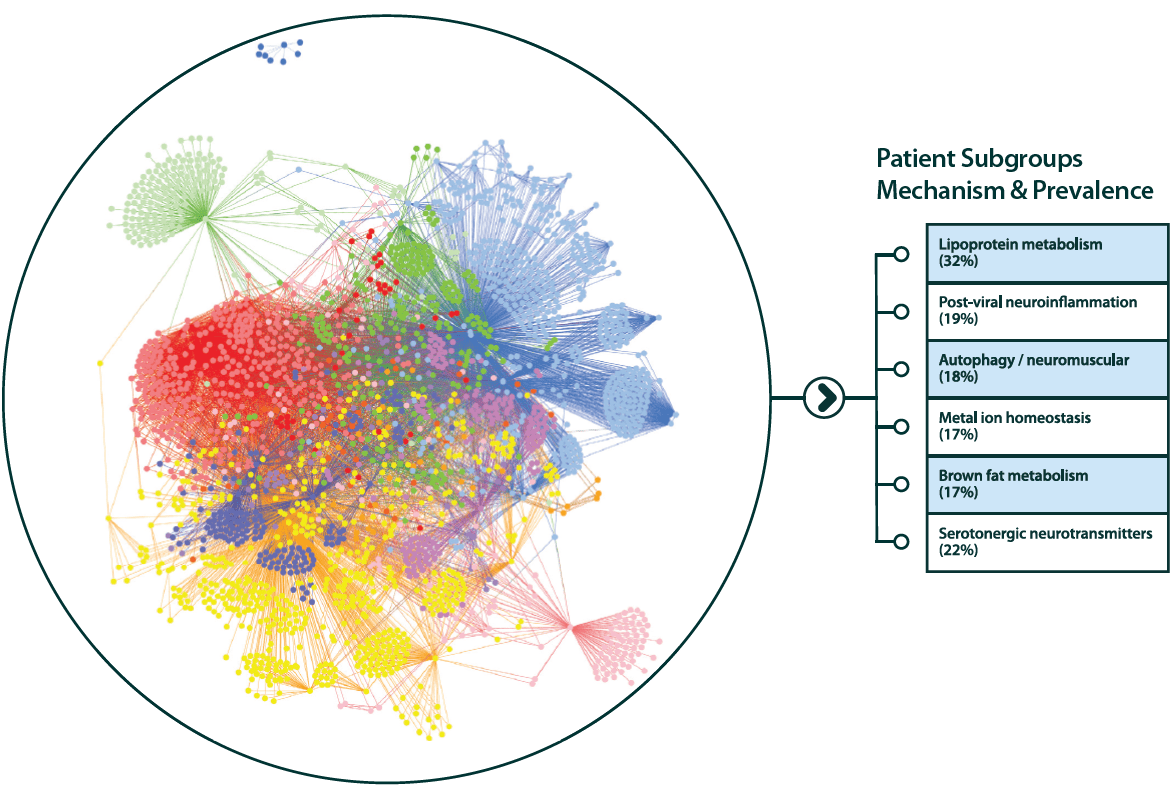
Mechanistic patient stratification of Alzheimer's disease
Our analysis found that only 32% of Alzheimer’s patients in a trial would respond to drugs targeting amyloid – not enough to achieve efficacy endpoints – highlighting the need to enrich trials with patients who will respond to treatments targeting a specific mechanism.
Biology
We produce unrivaled insights into causal biology and highlight which mechanisms are relevant to which patients, reproducibly, across multiple ancestries.
Biomarkers
We find mechanism- and drug response-based biomarkers to identify responders and support selected targets through development.
Efficacy
Our biomarkers inform trial design, predict efficacy, and enrich patient cohorts to maximize the likelihood of achieving efficacy endpoints in trials.
Diagnostics
These biomarkers are reduced to clinical practice in the form of low-cost genotypic tests, supporting product launch and enhancing market adoption.
Use cases
We map drug candidates to patient responders with mechanistic biomarkers that inform inclusion criteria, rescue unsuccessful trials, and de-risk each phase of clinical development.
Precision trial design
Our mechanism-based patient stratification biomarkers inform inclusion criteria for the design of mechanistically targeted clinical trials.
These biomarkers identify the broadest set of patients most likely to benefit from a drug candidate and are used as a simple genotypic inclusion test to recruit patients - maximizing the likelihood of positive readouts.

Efficacy trial rescue
We analyze drug response data from trials that missed efficacy endpoints, to find drug response biomarkers associated with strong responders.
This allows the option to redesign a subsequent trial and reposition the therapy as a precision medicine for that subgroup of patients using the patient stratification biomarkers as a complementary diagnostic tool.

Indication selection
Is there another indication in which your asset might be more successful?
We scan over 60 common diseases and their endotypes for causal links of a target’s mechanism of action to clinically relevant patient subgroups, finding the best candidate-indication pairing based on the prevalence of a chosen target’s mechanism in primary and/or secondary indications.
Let's talk
Patients are counting on us
We work with clinical development teams in biotech, pharma, and CROs to integrate mechanistic biomarkers into clinical programs across complex indications.
Contact us to explore how we can accelerate your development programs and increase your probability of success.