Precision Neuroscience
Extending precision medicine into neurological, psychiatric and neurodegenerative diseases
In this article, we explain how we're overcoming major challenges in understanding complex disease biology to achieve precision medicine in CNS conditions and how the new era of precision neuroscience is set to benefit patients and our biopharma partners who are investing in the field to develop better treatments for neurological and neuropsychiatric diseases.
Neurological and neuropsychiatric disorders, which include Alzheimer's disease, ALS, Parkinson's disease, schizophrenia, depression, and anxiety disorders, are a leading cause of disability and fatality worldwide, accounting for 17% of global deaths and 10% of disability-adjusted life-years (GBD 2015 Neurological Disorders Collaborator Group, 2017).
Achieving precision neuroscience requires a different way of thinking about disease biology...
The prevalence of these diseases has grown substantially in recent decades due to an increasing global population, longer life expectancies and increasing morbidity with predisposing risk factors. This now represents one of the most challenging and costly burdens to health and social care systems and societies worldwide.
The development of effective new disease-modifying treatments in neurodegenerative and neuropsychiatric disorders has been hindered by their inherent genetic complexity, environmental influences and clinical variability. Precision medicine approaches to neurological diseases, such as the use of tofersen to treat patients with specific SOD-1 mutations in ALS, are in their infancy but are fundamental to making meaningful progress and creating effective new therapeutic options in diseases such as ALS, Alzheimer’s and schizophrenia, which still have huge unmet medical need.
To find new ways of diagnosing and treating complex diseases we first must understand the mechanisms underpinning their key pathological changes, how these relate to different patient subgroups, and which drugs might be useful in reducing their effects – this is the basis of precision neuroscience.
Enabling Precision NeuroscienceWatch our presentation at the CNS Summit, which was awarded this year's Innovation Showcase prize. |
Guiding Successful CNS Drug Discovery & Development
The 20 years since the Human Genome Project has seen transformational advances in the molecular understanding of cancers and rare genetic diseases, leading to genetically informed, personalized selection of therapies and massively improved outcomes for many patients.
This is comparatively easier to accomplish in these diseases because they are relatively monogenic. Mutations in a gene may cause structural changes in its protein product, which can lead to a loss of function and consequently to the onset of a disease. While this is an oversimplification, usually all of the patients who share a specific mutation in their tumor can be treated in a similar fashion as they will share this common mechanistic etiology for their disease.
Precision neuroscience has similar potential to revolutionize neurological drug development, but it requires a different way of thinking about disease than we usually apply in oncology. With complex psychiatric and neurodegenerative disorders there may be multiple ways to arrive at the same clinical diagnosis – patients may have fundamentally different causes of disease, but these end up presenting clinically with similar symptoms, so they get the same diagnosis.
Before we can effectively study these diseases, we therefore require a deep understanding of the mechanistic stratification of patients suffering from them. To do otherwise risks missing novel targets or overfocusing on the most obvious GWAS associations, such as that linking the APOε4 gene to Alzheimer’s, in the expectation that we can force a “one-drug-fits-all” solution. The fact that 100+ clinical trials in Alzheimer’s have failed to demonstrate clinical efficacy should warn us that this is too simplistic an approach in neurological diseases.
This is wholly unsurprising. If a disease has more than one mechanistic etiology, it’s clear that the maximum efficacy that any drug will ever be able to demonstrate is equivalent to the prevalence of that disease mechanism in the patients recruited to a clinical trial. This is an obvious, accepted principle in oncology, and yet we keep expecting much more complex neurological diseases to behave in a much simpler ‘monogenic’ fashion. This has been the real failure of CNS drug discovery.
In reality, this is as much of an opportunity as it is a failure. It’s not accurate to infer that all of the drugs tested in these 100+ clinical trials did not work – in reality some of them probably did work (and there are certainly anecdotal reports of patient benefit for many), but just not in sufficient patients to demonstrate the level of clinical efficacy required when they were tested across the whole population.
A Better Understanding of Neurological and Neuropsychiatric Disease Biology
Neurological diseases have been particularly challenging to treat due to their complexity; they are multifactorial in nature, arising from interactions between multiple genes and other clinical, epidemiological, and environmental factors, with significant individual variability in the underlying genetics and biology. They also involve multiple mutations in non-coding regions of DNA – the “dark genome” which makes up around 98% of our DNA. Mutations in the dark genome affect gene regulation rather than coding, exerting a positive or negative impact on the expression of genes that may be related to disease processes. Due to this complexity, and in spite of major research efforts, effective preventative and disease-modifying treatments are yet to materialize for diseases such as ALS, Alzheimer’s and Parkinson’s.
Central Nervous System (CNS) treatments in general have higher failure rates than non-CNS drugs, most often because of a lack of significant evidence of clinical efficacy and/or an unacceptable ratio of serious adverse events to demonstrable patient benefit. The development and post-development regulatory review times for CNS drugs can be significantly longer due to their complexity, although regulators such as the FDA are beginning to take a more flexible approach through limited/conditional approvals1.
Precision neuroscience approaches need to integrate genomics, clinical, phenotype, and epidemiological data with human biology to better understand the etiologies of these complex diseases and mechanistically stratify patient subgroups. Only by stratifying CNS disorders more precisely can we identify novel druggable targets and distinguish the patient subgroups who will benefit from them using mechanistic biomarkers.
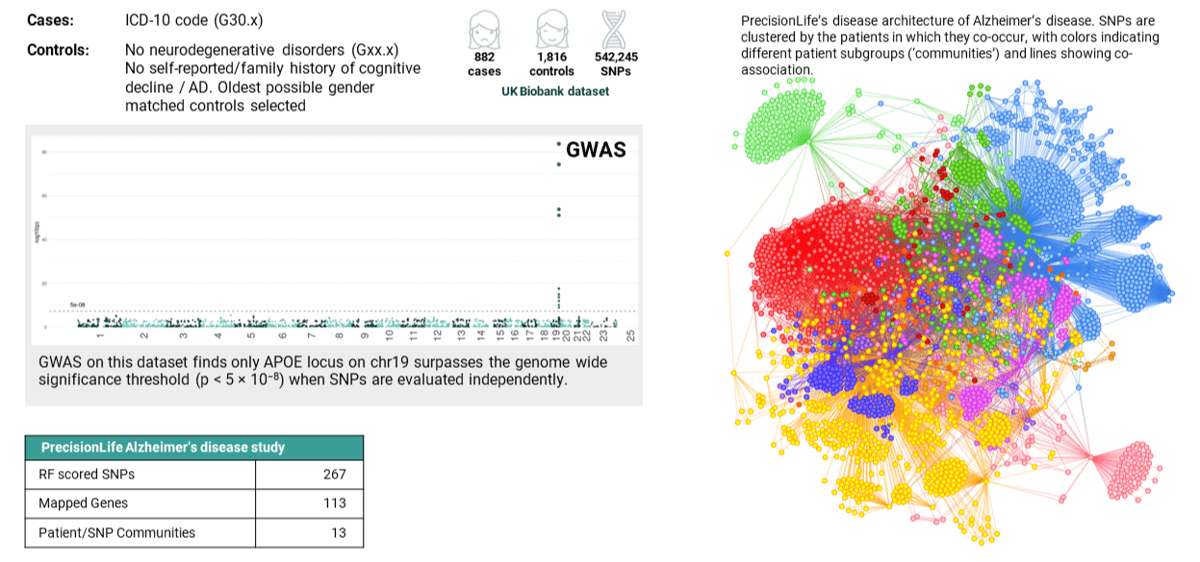
Figure 1. Comparing results: in an analysis of the same UK Biobank Alzheimer’s disease cohort, GWAS identified a single gene (left) and PrecisionLife identified 13 patient communities comprising 267 SNPs mapped to 113 genes (right).
Mechanistic Patient Stratification in Complex Diseases
PrecisionLife’s combinatorial approach allows us to routinely use our platform to stratify patient populations into clinically relevant subgroups based on their distinct mechanistic etiologies.
As an example, PrecisionLife analyzed genomic data from 900 patients diagnosed with Alzheimer’s disease against healthy controls with no family history of the disease. While a traditional GWAS analysis of the same dataset only identified the APOε4 locus, the PrecisionLife platform identified 267 SNPs that are highly associated with the risk of developing early-onset Alzheimer’s disease.
This additional sensitivity of the PrecisionLife platform comes from three main sources:
-
We identify the non-linear effects of interactions between SNPs/features as well as the traditional linear additive (single SNP/feature) associations found by GWAS
-
We perform our analysis on every SNP/feature in the dataset – we do not use a priori data reduction methods like LD clumping before our analysis
-
We can identify combinations that include multi-modal data features (such as clinical, epidemiological or expression) as well as (or instead of) the genomic data
By clustering patients who in this case shared the same disease-associated SNPs, we found six major subgroups of patients with distinct mechanisms underpinning their disease. Lipoprotein metabolism (the target for most current Alzheimer’s drug development programs) is the main driver of disease in this population, but crucially it is only relevant to about 1/3 of these patients.
This defines the upper bounds of clinical efficacy that is likely to be demonstrable in a Phase III trial if the patients are chosen at random because they have a clinical diagnosis of Alzheimer’s and meet other non-genetic inclusion criteria.
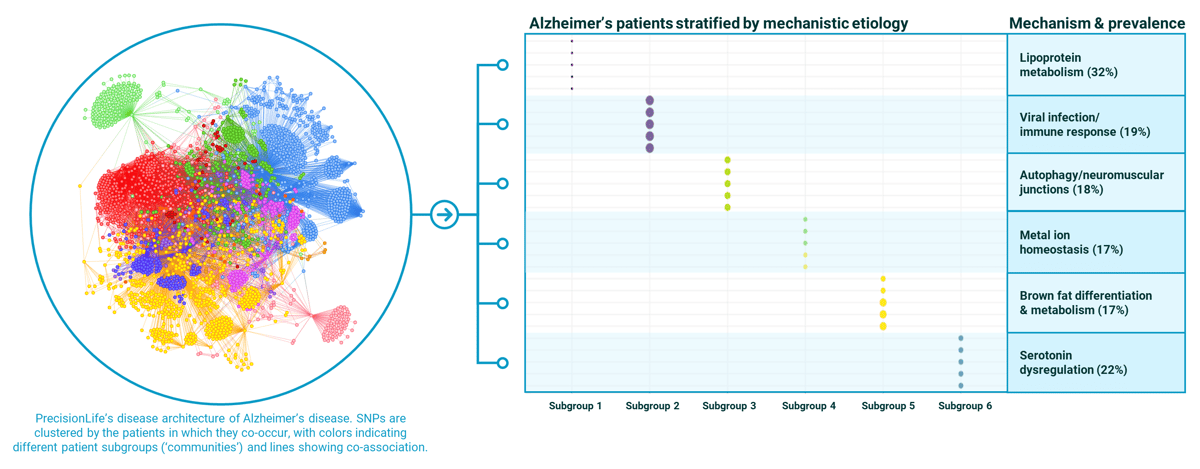
Figure 2. PrecisionLife stratification of Alzheimer’s disease population into 6 mechanistically distinct patient subgroups with case prevalence (%) for each mechanism in the Alzheimer’s population shown.
Generating Precision Biomarkers
Mechanistic stratification changes our view of the disease from being a single diagnostic label into a series of subgroups, each of which has a slightly different disease etiology, each of which will therefore benefit from a different drug targeting a different mechanism of action.
With this new level of insight…
-
we found new subgroups of Alzheimer's disease patients each with a different mechanistic etiology with >15% prevalence, with multiple novel targets with strong genetic linkage for each patient subgroup,
-
we generate mechanistic patient stratification biomarkers which can be used as inclusion criteria for recruitment of participants into the clinical trial to enable the design of smaller, faster and more targeted trials that are more likely to read out successfully,
-
complementary diagnostics can be developed based on those same patient stratification biomarkers – allowing clinicians to identify the right therapy for a specific patient and to support the launch and prescription of a new medication, especially in extended open-label trials.
Even stratified, these are still huge markets – Alzheimer’s and other dementias are estimated to directly cost US health systems $345B per year and this will rise by 2050 to $1 trillion2. Even 15% of the Alzheimer’s market is worth >$5B/year on a willingness to pay basis.
All this is of huge interest when you're putting a new drug on the market and working inside an outcomes-based remuneration model.
However, for those pharmaceutical companies developing CNS treatments without having these patient stratification biomarkers to identify the clinical trial recruits who will benefit from a mechanism, the upper limit of clinical efficacy that they can demonstrate is greatly compromised, and their programs are more likely to fail.
As well as condemning $100Ms of R&D investment to be spent in vain and billions of dollars of forecast revenues to be unrealized, this also means that CNS drugs that could in fact have been highly effective for many patients will not make it to market.
Precision neuroscience approaches will be essential to overcome the fundamental challenges of such complex, heterogenous and multi-factorial diseases.

Accelerating Progress in Precision Neuroscience
This precision neuroscience approach is unique to PrecisionLife and proving to be highly effective in even the most complex neurological and neuropsychiatric indications, such as ALS, Alzheimer's, Schizophrenia, and over 45 other chronic diseases.
We are using our mechanistic patient stratification biomarkers to support biopharma partners with target discovery, indication selection, clinical trial design, clinical trial rescue, and companion diagnostics, and we are interested in partnering with companies that share our ambition to develop new treatments for people with unmet clinical needs.
Find out more
See how we're driving precision medicine in CNS disorders and view more precision neuroscience research from PrecisionLife.
References
1. https://www.nature.com/articles/d41586-023-01039-4
2. https://www.ajmc.com/view/economic-burden-of-alzheimer-disease-and-managed-care-considerations
Sign Up


