Increasing Healthspan
Predicting & Preventing Chronic Diseases with Precision Medicine
Contents
This paper describes how building deeper understanding of the drivers of complex disease biology, embodied in highly predictive, mechanism based patient stratification biomarkers, leads to a new generation of better, more accurate diagnostics tools that are more personalized and clinically actionable.
Using these to systematically uncover actively protective disease resilience mechanisms that work to prevent complex diseases, and targeting these with repurposed drugs and novel modalities, will lead to new prophylactic drugs and therapeutic mRNA vaccines that can delay onset and prevent progression of major chronic diseases.
Healthspan - the period of life spent in good health, free from chronic diseases and disabilities of aging
How Healthy is Healthcare?
Healthcare is a $10 trillion industry globally, costing £292B (11% of GDP) in the UK1 and $4.8 trillion in the US (18% of GDP)2. These costs are growing at a long-term average of around 2% p.a. above GDP3, in large part due to earlier onset of chronic diseases and increased prevalence of older patients with multiple chronic conditions – diabetes, respiratory, cardiovascular, dementia etc. These complex, chronic diseases affect billions of patients and account for over 80% of healthcare spending globally as they are difficult to diagnose, expensive and often poorly managed.
At age 65 the cost of delivering healthcare spirals, to an average of 5x the cost of below-65 patients. This cohort of over-65s is expected to grow by 35% and the number of over-80s to double in the next 20 years in the UK4. In developing countries, the burden of chronic disease is often appearing 5-10 years earlier than the UK or US5. In short, healthcare as we know it today is becoming unaffordable in all major economies, as is the economic burden of disease and the overspill of chronic disease management into social care.
Spending more on treating sick people doesn’t work – the US spends double other countries on healthcare but its outcomes are no better – it is consistently mid-table in outcomes vs other major economies6.
New proactive health maintenance approaches are required to accurately identify at-risk people, and diagnose, prevent and/or better manage the increasing burden of complex chronic diseases7,8.
Biomarkers of Complex Disease Biology
Precision medicine approaches have transformed oncology9, but have had much less impact in highly prevalent and expensive chronic diseases, such as mental health disorders, respiratory diseases, dementias, endometriosis, chronic kidney disease (CKD), irritable bowel syndrome (IBS).
Major complex diseases such as Alzheimer’s (figure 1) are more heterogenous and polygenic than most cancers10, meaning they have a larger need for precision solutions, but also that the traditional precision medicine toolkit (whole genome sequencing, GWAS and PRS) doesn’t work as well and have had less impact in these diseases11,12.
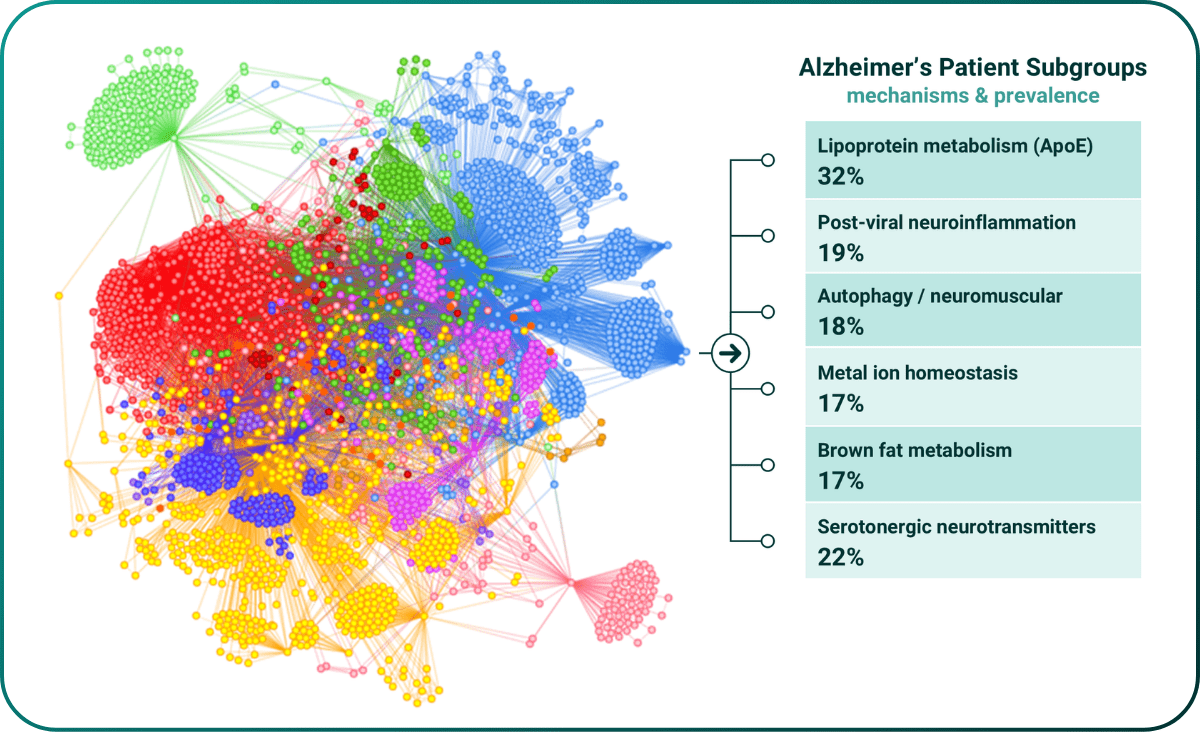
Figure 1. Mechanistic stratification of early onset Alzheimer’s disease population from UK Biobank.
Advances in combinatorial analysis13 have led to much deeper understanding of disease biology in cryptic diseases with 100% unmet diagnostic and therapeutic need, such as ME/CFS (shown below).
Prior to PrecisionLife’s ME/CFS study14, there were no known genetic associations from any GWAS study. To check clinical relevance, the hypothesis free mechanistic stratifications from PrecisionLife’s combinatorial analysis were compared against the phenotypic presentations of the patients in a subgroup to check they do have the predicted symptoms.
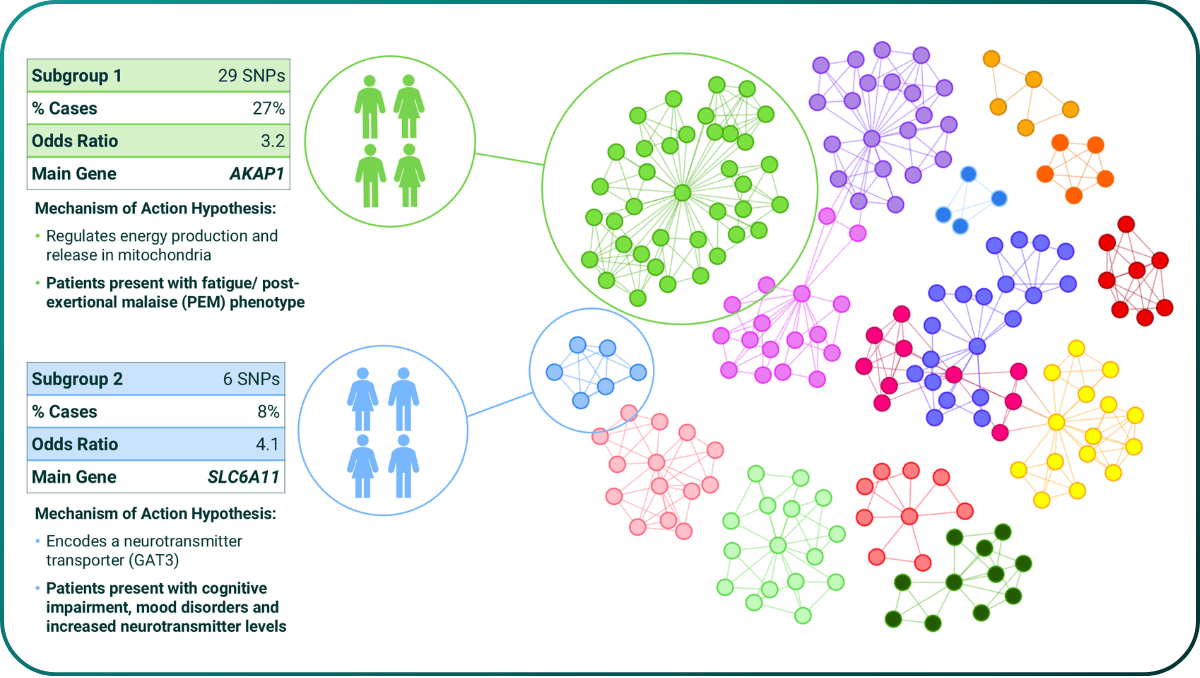
Figure 2. Mechanism based patient stratification of ME/CFS (circles are SNPs, lines indicate co-association in patients and colours represent different mechanistic causes of disease in that patient subgroup). All MoA hypotheses were validated against clinical records.
Early Diagnosis & Prevention
This mechanistic stratification captures both linear and non-linear disease biology and is highly predictive of disease. The study above reported 199 significant SNPs in 84 combinations, with a combined odds ratio for predicting ME/CFS disease of 8.9 – equivalent to monogenic risk factors such as BRCA1. It is also simple to reduce to practice as a saliva or blood based genotyping test (a Mechanostic™ test) capturing both disease risk and the patient’s underlying mechanistic causes of disease, and therefore their likely effective therapies.
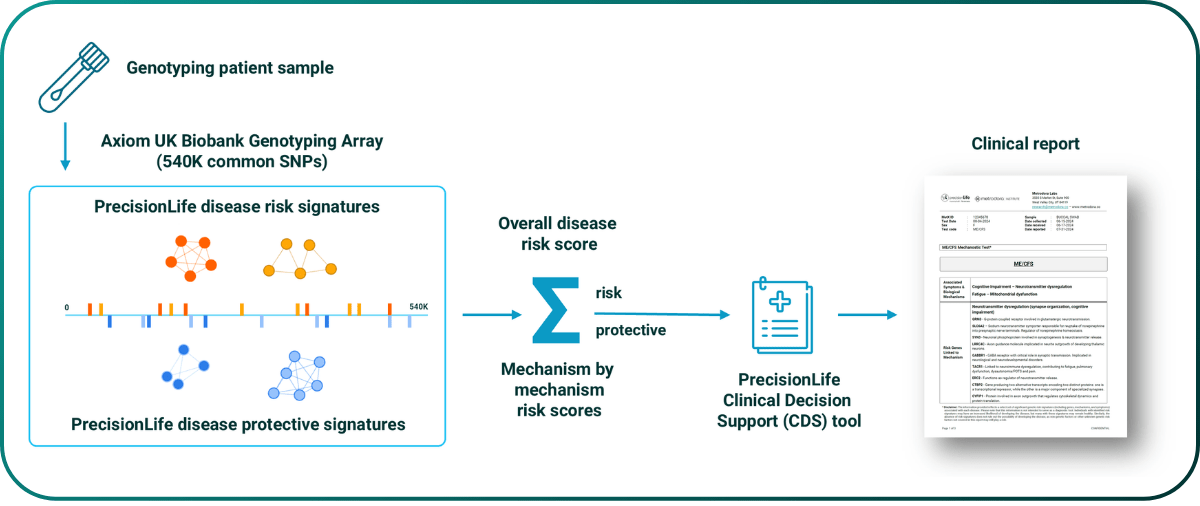
Figure 3. Reduction to clinical practice of PrecisionLife’s ME/CFS mechanostic test.
Our partners at Metrodora Institute are currently clinically validating this test in a 1,000 patient trial15. These tests can be used for differential triage, to identify the likelihood of a patient having one disease vs another (which may have similar symptoms), as well as for personalized therapy selection, based on the specific mechanistic cause(s) of disease in that patient.
Genotype-based tests have advantages over WGS and metabolomic based tests including ease of use, cost and prediction of disease risk prior to symptoms.
Case Study: Endometriosis
Many highly prevalent diseases are still poorly diagnosed. This diagnostic delay means patients suffer for years with debilitating pain, fatigue, mood disorders and other serious symptoms that prevent them fulfilling their role in society. This has a massive impact on patient lives and is a huge waste of clinical resources in excess clinical visits, ineffective treatments and avoidable co-morbidities.
Endometriosis is one such progressive debilitating disease affecting 10% of women of childbearing age (~190 million women worldwide16). Patients present with a complex variety of disease symptoms, severities and co-morbidities (e.g. infertility). This heterogeneity means there are no accurate non-invasive diagnostic tests or effective treatment options. It currently takes 8-10 years in the US and 10+ medical consultations on average for women to receive a definitive diagnosis, which is only confirmed by invasive, risky and painful laparoscopic surgery. Its impact on sufferers and the socioeconomic and direct clinical costs are huge17,18.
reducing diagnosis from 8 years to 2 weeks
Mechanostic biomarker driven tests can identify at-risk patients non-invasively, quickly and cheaply, and identify the best disease management or therapeutic options for them (including repurposed drugs).
This test could identify other causes of deep pelvic pain (e.g., IBS) avoiding the cost and risks of the 35% of laparoscopic surgeries that have a negative outcome, reduce initial diagnosis from 8 years to 2 weeks, and reduce incidence of disease associated co-morbidities, e.g., mental health, infertility, and ovarian cancer.
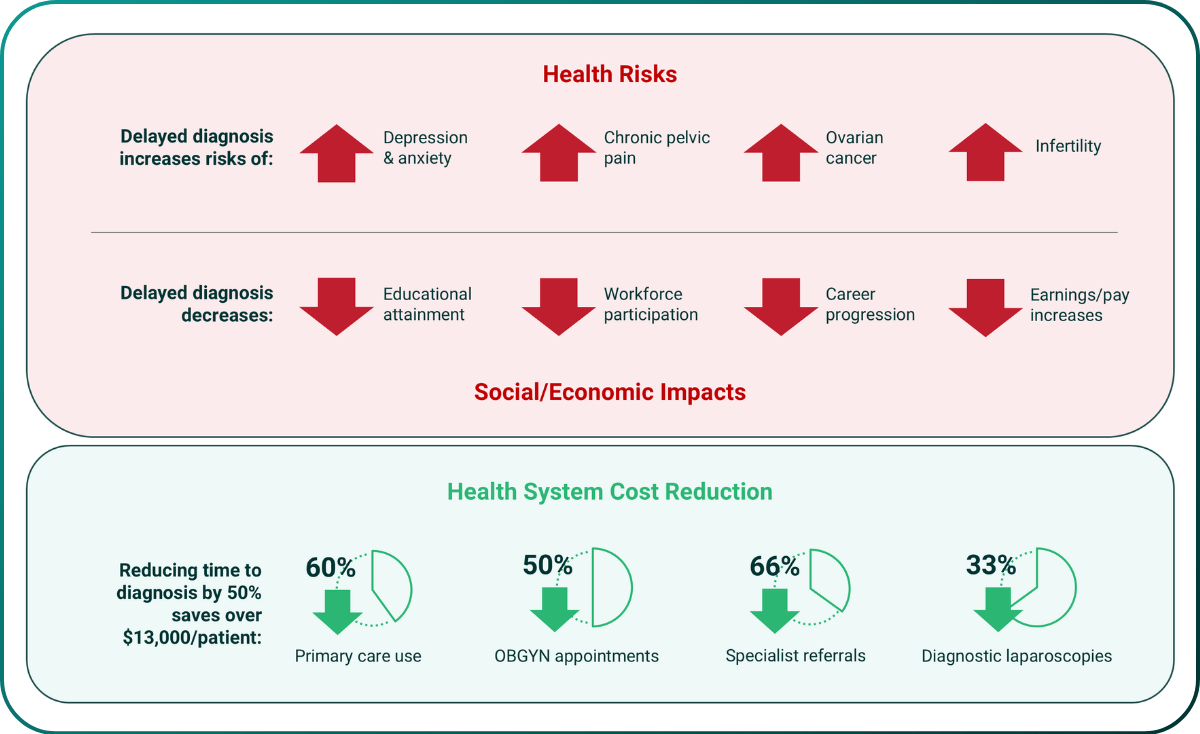
Figure 4. Costs, health & socioeconomic impacts of average 8-10 year diagnostic delays in endometriosis.
Case Study: Chronic Kidney Disease in Type-II Diabetes Patients
Type-II diabetes costs $413 billion (2% of GDP) in the US19 and is growing rapidly. 80% of that cost comes from disease complications including renal failure, neuropathy & blindness, ulcers & amputations, and stroke. In the UK, diabetes leads to 2,990 heart failure cases, 930 strokes, 660 heart attacks and 184 amputations weekly20.
Precision medicine tools can play a key role in early identification of patients most at-risk of developing specific complications, providing them and their clinicians with actionable insights, and helping target interventions to them before they experience the most serious complications.
Diabetes associated renal failure is hugely expensive and life-limiting due to the need for dialysis and/or transplant. Predicting at-risk patients at initial diagnosis provides an average 5-year prodromal window into which tailored monitoring, lifestyle changes and/or therapeutic interventions for weight loss, glycaemic control or prophylactic kidney protection can be deployed. Impacting just 25% of these patients could save $40B/yr in the US.
predicting risk early in 25% of patients could save $40B/year in the US alone
At the point of initial diagnosis PrecisionLife’s risk model predicts a patient’s likely complications trajectory (AUC=0.78, below), and can guide treatment selection, e.g., identifying patients most likely to benefit from treatment with Kerendia to reduce progression of CKD in type-II diabetics. Prescribed earlier this may act prophylactically to limit renal damage and reduce/avoid any symptoms of renal failure even in high-risk patients.
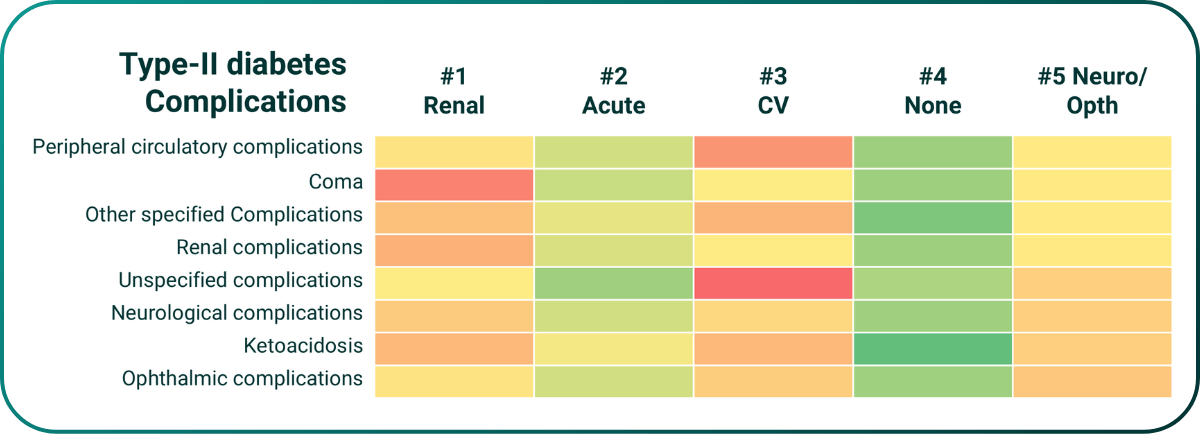
Figure 5. PrecisionLife model predicting disease complication risks in type-II diabetes.
Actively Protective Biology
A key enabler for increasing healthspan in a rational, scientific evidence led manner is to find components of cellular biology that act to resist disease pressures and stop a flip over into a disease phenotype. When supported and/or stimulated, these resilience mechanisms could work to prevent disease and/or reduce the severity or progression rate of key pathophysiological processes.
PrecisionLife is uniquely able to take advantage of the highly predictive disease risk models to systemically identify actively protective biology. By screening a large population who don’t have the disease against this risk model, we find a cohort of people who in spite of having all of the risk signatures, exposure triggers and being old enough that they should have the disease, have not actually been affected by it. This cohort can be inferred to be enriched in actively protective factors that are preventing them from developing the disease.
Removing their disease associated risk genes leaves behind just those unique to this protected cohort. These appear to be protecting against the development of the disease, even in patients otherwise at very high risk. This is a new class of actively protective targets that can be drugged by existing modalities, or via stimulation of the production of protective proteins using therapeutic mRNA or DNA vaccines.
With access to suitable data, this approach could be used to identify more broadly geroprotective factors.
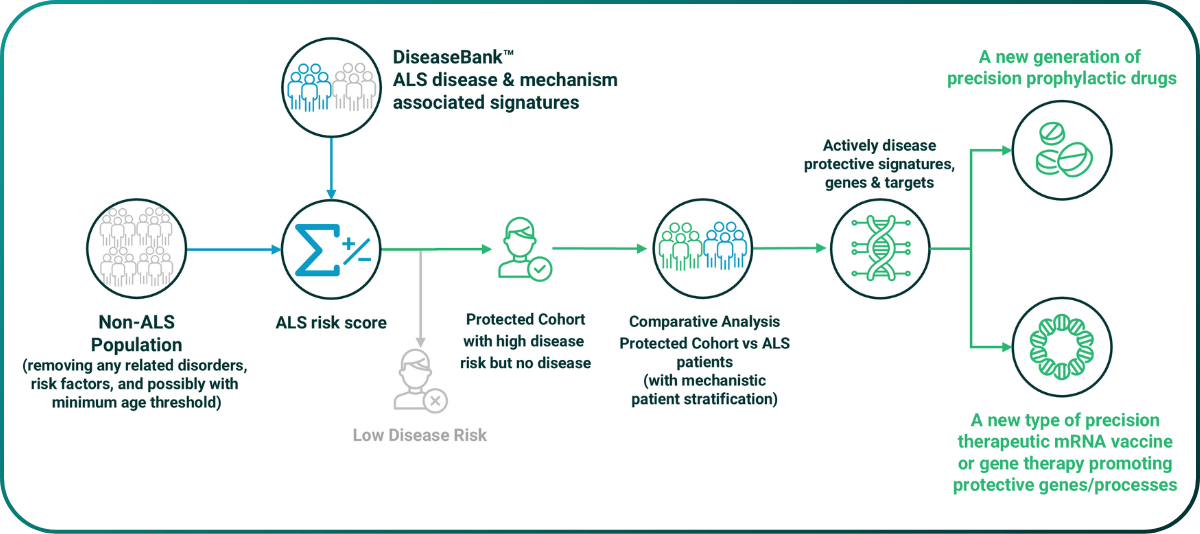
Figure 6. Schematic workflow for systematic identification of actively protective biology targets.
Case Study: ME/CFS
In the ME/CFS study we identified a series of actively protective genes, all of which are associated with highly credible protective processes modulating known pathophysiology. In the two independent runs shown below the same gene involved in tyrosine catabolism (a component of dopaminergic biology) was found to be protective. There is anecdotal clinical evidence from Metrodora to support this finding.
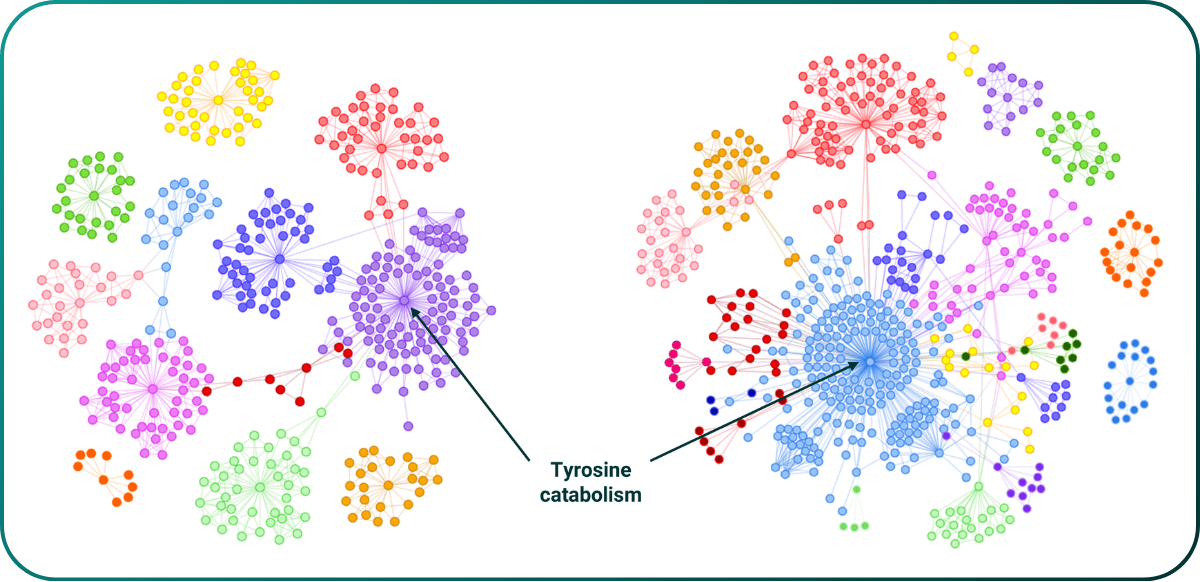
Figure 7. Identification of a replicated actively protective gene involved in producing protective GPR35 agonist metabolites in ME/CFS.
Case Study: ALS
Our ALS actively protective analysis highlighted several genes, including GRIP1, which has previously been associated with rare cases of ALS reversal21, and MTRR, which codes for an enzyme that is central to the role of vitamin B12 within the cell. This observation supports findings from PrecisionLife’s causative analyses which saw signal from three other genes linked with cobalamin/vitamin B12 uptake/transport – CUBN, LRP2 and CD320.
Vitamin B12/methylcobalamin has previously been explored in cellular, animal and clinical studies for its role in ALS, with promising outcomes22. However there has not been any previous genetic basis to link it to ALS.
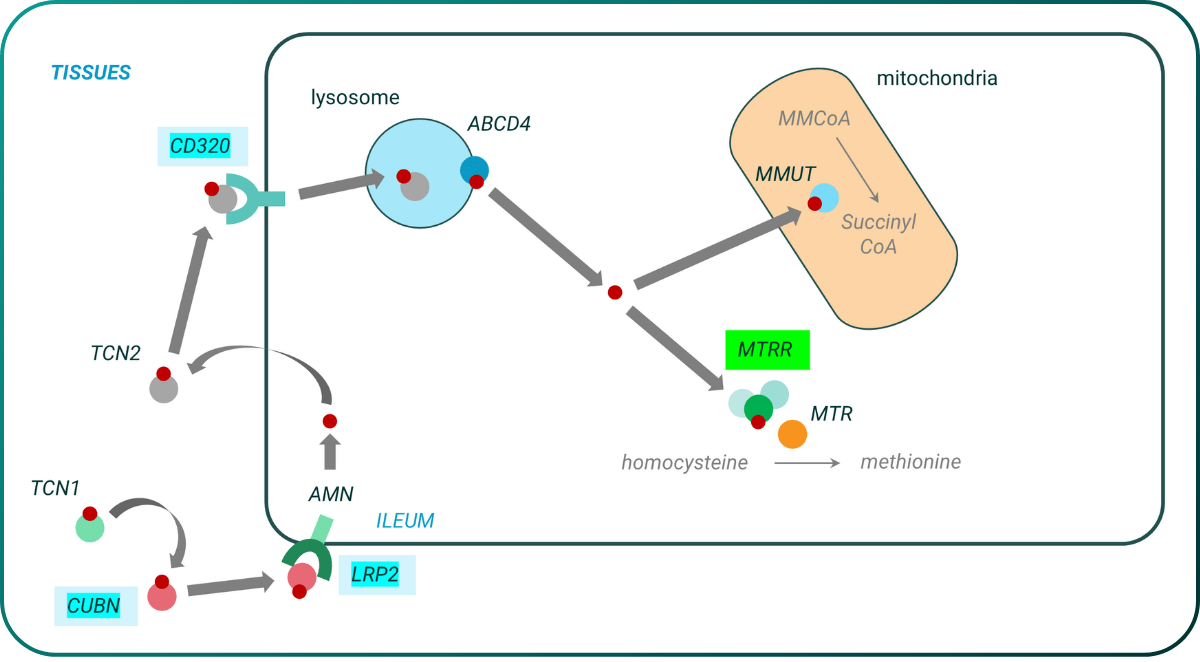
Figure 8. Identification of a replicated actively protective gene (MTRR) involved in uptake and function of cobalamin/vitamin B12 in ALS.
New Therapeutic Options
The new disease biology (risk & actively protective) generates multiple opportunities for novel drug programs and even potentially the development of evidence led combination regimens. It reinforces the importance of using mechanism related biomarkers to select the most likely responders for clinical trial recruitment, and this approach opens up a series of highly capital and time efficient new drug repurposing opportunities.
Drug Repurposing
For any novel target, PrecisionLife’s indication extension platform23 can be used to identify drug candidates that modulate that target in the right direction, regardless of its original indication. PrecisionLife has identified hundreds of such compounds that may well be useful for repurposing into patients.
This is the fastest way to identify novel therapeutic options for patients. In severe COVID-19 it resulted in PrecisionLife identifying dutasteride24, which has been shown to reduce infection severity and length by over 40% in double-blind randomized controlled trials25,26.
rapid 28-day clinical efficacy trials
Arm 2 of the Metrodora trial is designed to use generic drugs as tool compounds has identified. We are using the Mechanostic tests to identify n of 30 patient cohorts who share specific high-prevalence mechanistic defects that can be modulated by a small molecule generic drug. We are selecting phenotypes with a short (28/56 day) clinical readout. These are ideal for very small, fast and cost-effective efficacy trials of repurposing candidates. PrecisionLife has identified 9 suitable repurposing candidates in ME/CFS and long COVID.
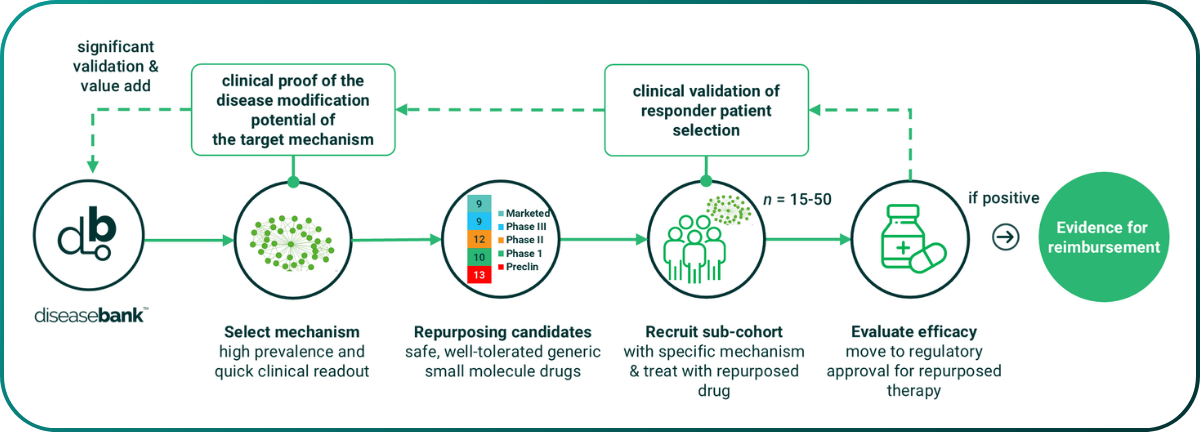
Figure 9. Arm 2 of the Metrodora clinical trial – selecting small mechanistically defined patient cohorts (n=30) with matched small molecule generic repurposing candidates for rapid clinical efficacy trials.
Caring for Healthcare
Proactive health maintenance tools are essential components for increasing healthspan, freeing patients and healthcare from the costs and burden of avoidable chronic disease. However, there are currently few tools that enable clinicians to move to predictive/preventative medicine due to a lack of capability to unlock the predictive signal in patient data and reduce the insights to clinical practice.
Multi-stage Triage for Chronic Diseases in Routine Clinical Care
Predictive screening and validation of disease risks at a population level can be achieved accurately and cost-effectively by combining two approaches enabled by new technologies:
Detection of ‘at-risk’ cohorts using text mining (small language models) of EHR and claims data for early symptom detection
Validation of a patient’s specific disease risks using Mechanostic tests
This will help identify disease risk early, and guide development of a personalized care path, which may include tailored monitoring, lifestyle changes and/or other actively protective or prophylactic therapies.
Optimizing the semantic profiles using real-world EHR diagnosis & outcomes data over time will improve the diagnostic performance of these systems. This will also reduce the potential for systematic bias and a lack of health equity inherent in externally trained systems that do not reflect the local patient population.
The development of such a system will not only bring direct cost/outcomes benefits but also create valuable new data products and opportunities for new revenue streams.

Figure 10. Proactive identification of ‘at-risk’ patient populations, validated by Mechanostic tests to identify optimal intervention strategy.
Primary Benefits
The primary benefit of a hybrid EHR / genetic diagnostics screening approach is to use very cost-effective and non-intrusive screening tools to reduce the time to detection and diagnosis of early disease in patients and to be able to offer personalized interventions that reduce or even avoid the onset of symptoms.
This has significant potential benefits in terms of reducing the cost of providing care while improving outcomes for patients, and also identifying cohorts who would benefit from specific therapies earlier.
It offers a route to improve health equity and evidence-led access to effective medicines for the cohorts most likely to benefit from them.
The use of genotypic diagnostics for triage purposes does not imply that full genetic counselling is required to be deployed in primary clinical care. In most cases, this would be used simply for effective triage and early referral to specialist clinicians.
detection of early disease & treatments that prevent the onset of symptoms
Secondary Opportunities
The routine use of genotyping or low-pass sequencing as an integral part of cost-effective clinical care offers the opportunity to build at minimal additional cost an ethically sourced, diverse, research consented patient population with connection to longitudinal EHR, claims, prescriptions and outcomes data and with potential for patient follow-up. This would be a very attractive and valuable resource for genetic research studies and clinical trials recruitment.
Access to clinical trials is both a significant revenue source and provides the highest quality of care for patients. The broader distribution of genetic ancestries recruited is being encouraged strongly by regulators for clinical trial design and this again improves health equity. Smaller biomarker driven clinical trials can be used to support drug repositioning studies in patients, which get effective medicines to patients quicker, and which may offer further revenue and IP royalty sharing opportunities.
References
1 ONS Healthcare expenditure, UK Health Accounts provisional estimates: 2022 and 2023.
2 CMS NHE Fact Sheet.
3 Keehan SP, Cuckler GA, et al. CMS National Health Expendiure Projections, 2019-28: Expected Rebound In Prices Drives Rising Spending Growth. Health Aff (Millwood). 2020 Apr;39(4):704-714. https://doi.org/10.1377/hlthaff.2020.00094.
4 Our Ageing Population | The State of Ageing 2023-24, Centre for Better Ageing (2024).
5 Alqahtani, B., Elnaggar, R.K., et al. National and regional prevalence rates of diabetes in Saudi Arabia: analysis of national survey data. Int J Diabetes Dev Ctries 43, 392–397 (2023). https://doi.org/10.1007/s13410-022-01092-1.
6 U.S. Health Care from a Global Perspective, 2022: Accelerating Spending, Worsening Outcomes.
7 Prosperity Through Health: The Macroeconomic Case for Investing in Preventative Health Care in the UK, 2024, Tony Blair Institute for Global Change.
8 The Economic Case for Protect Britain, a Preventative Health Care Delivery Programme, 2024, Tony Blair Institute for Global Change.
9 Rulten SL, Grose RP, Gatz SA, Jones JL, Cameron AJM. The Future of Precision Oncology. Int J Mol Sci. 2023 24(16):12613. https://doi.org/10.3390/ijms241612613.
10 https://precisionlife.com/driving-precision-medicine-in-cns-disorders.
11 Tam V, Patel N, Turcotte M, Bossé Y, Paré G, Meyre D. Benefits and limitations of genome-wide association studies. Nat Rev Genet. 2019;20(8):467–84. https://doi.org/10.1038/s41576-019-0127-1.
12 Horesh Bergquist S, Lobelo F. The limits and potential future applications of personalized medicine to prevent complex chronic disease. Public Health Rep. 2018;133(5):519–22. https://doi.org/10.1177/0033354918781568.
13 Gardner, S. Combinatorial analytics: an essential tool for the delivery of precision medicine and precision agriculture. Artif Intell Life Sci. 2021;1:100003. https://doi.org/10.1016/j.ailsci.2021.100003.
14 Das, S., Taylor, K., Kozubek, J., Sardell J., Gardner, S. Genetic risk factors for ME/CFS identified using combinatorial analysis. J Transl Med 20, 598 (Dec 2022) https://doi.org/10.1186/s12967-022-03815-8.
15 PrecisionLife and Metrodora Institute share first insights with ME/CFS, Long COVID participants in MetX study.
16 Endometriosis Fact Sheet 2023 (WHO) https://www.who.int/news-room/fact-sheets/detail/endometriosis.
17 Armour M, et al. The cost of illness and economic burden of endometriosis and chronic pelvic pain in Australia: A national online survey. PLoS One. 2019 Oct 10;14(10) https://do.org/10.1371/journal.pone.0223316.
18 Surrey E, et al. Impact of Endometriosis Diagnostic Delays on Healthcare Resource Utilization and Costs. Adv Ther. 2020 Mar;37(3):1087-1099. https://doi.org/10.1007/s12325-019-01215-x.
19 Parker ED, Lin J, et al. Economic Costs of Diabetes in the U.S. in 2022. Diabetes Care. 2024 Jan 1;47(1):26-43. https://doi.org/10.2337/dci23-0085.
20 Hex N, MacDonald R, Pocock J, et al. Estimation of the direct health and indirect societal costs of diabetes in the UK using a cost of illness model. Diabet Med. 2024; 41:e15326. https://doi.org/10.1111/dme.15326
21 Crayle JI, Rampersaud E, et al. Genetic Associations with an Amyotrophic Lateral Sclerosis Reversal Phenotype. Neurology. 2024 Aug 27;103(4):e209696. https://doi.org/10.1212/WNL.0000000000209696.
22 Neuroprotective effect of ultra-high dose methylcobalamin in wobbler mouse model of amyotrophic lateral sclerosis Ikeda, K et al. Journal of the Neurological Sciences 2015 354(1), 70-74.
23 Das S, Taylor K, Beaulah S, Gardner S. Systematic indication extension for drugs using patient stratification insights generated by combinatorial analytics. Patterns (N Y). 2022 Jun 10;3(6):100496. https://10.1016/j.patter.2022.100496.
24 Taylor, K., Das, S., Pearson, M., Kozubek, J., Pawlowski, M., Jensen, CE., Skowron, Z., Møller, GL, Strivens, M., Gardner, S. (Jun 2020). Analysis of genetic host response risk factors in severe COVID-19 patients Preprint at medRxiv. https://doi.org/10.1101/2020.06.17.20134015.
25 Samuel RM, Majd H, et al. Androgen Signaling Regulates SARS-CoV-2 Receptor Levels and Is Associated with Severe COVID-19 Symptoms in Men. Cell Stem Cell. 2020 Dec 3;27(6):876-889.e12. https://doi.org/10.1016/j.stem.2020.11.009.
26 Cadegiani FA, McCoy J, et al. Early Antiandrogen Therapy with Dutasteride Reduces Viral Shedding, Inflammatory Responses, and Time-to-Remission in Males With COVID-19: A Randomized, Double-Blind, Placebo-Controlled Interventional Trial (EAT-DUTA AndroCoV Trial - Biochemical). Cureus. 2021 Feb 1;13(2):e13047. https://doi.org/10.7759/cureus.13047.


