We believe in optimized portfolios informed by the deepest biology insights to accelerate approvals of life-changing treatments
Generate new value from existing assets
Over 90% of clinical drug candidates for chronic diseases never achieve market approval while many of those that are approved face a looming patent cliff.
Yet, many of these programs still have long-lasting clinical and commercial value which can be unlocked with precision repositioning and label expansion – targeting the patients who are most likely to respond to the mechanism of action.
This is a new era of the precision optimized R&D portfolio.
>33% new revenue
via precision indication expansion, label extension and out-licensing with higher success rates and ROI.
Maximize returns with genetic evidence
Our unique understanding of disease biology and accurate prediction of which patients a drug targeting a specific mechanism is going to work enables us to maximize R&D ROI.
We identify and validate opportunities to expand development candidates, stalled assets, and approved drugs into new indications and sub-populations that no other approach is capable of finding.
Accelerated approvals
With just the name of a target and its primary indication, our platform finds matches with clinically relevant patient groups in secondary indications based on the molecular mechanism of their disease or drug response.
We rapidly evaluate the clinical and commercial potential of each opportunity and support them with detailed data packages including novel biomarker IP to support and accelerate clinical development, approvals, and market adoption.
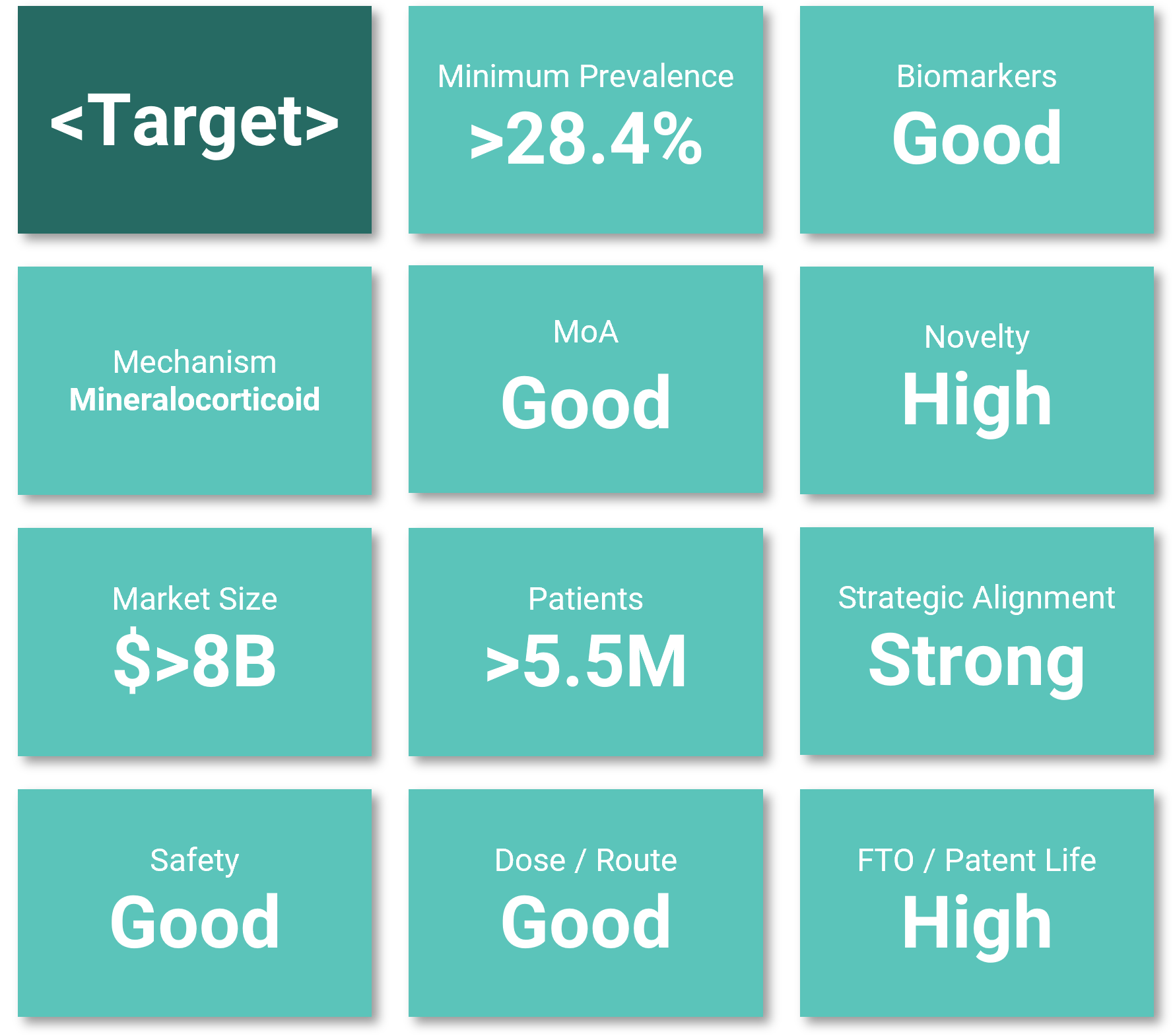
Portfolio assets selected
You provide the names and indications of the targets to assess.
We rapidly evaluate their potential in primary indications and align them with relevant disease mechanisms across more than 60 chronic conditions.
Opportunity mapping
We systematically identify and validate where each asset’s mechanism of action aligns with unmet patient needs.
This includes assessing genetic drivers, biological causality, and clinical relevance - highlighting both primary and secondary indication opportunities.
Strategic value assessment
We quantify the additive value of each opportunity with supporting data packages.
These include stratification biomarkers, efficacy potential, prevalence, commercial opportunity, and fit for in-/out-licensing or label expansion.
Acceleration-ready assets
We deliver high-confidence data packages with novel IP, mechanism-based biomarkers, and evidence to support trial design and regulatory engagement.
Each asset is de-risked, optimized for clinical success, and ready for progression or partnership.
Partner to optimize your portfolio
Partnering with PrecisionLife can be transformative to your portfolio, finding new, hidden opportunities to create additive value from R&D.
We inform portfolio strategy in multiple ways:
Indication expansion
Our disease biology insights across over 60 indications are used to identify and validate effective new purposes for existing drug candidates and on-market products with more innovation and less bias than alternative methods.
We provide detailed data packages that enable IP filings, including new method of use patents linking the drug to a second indication.
These are supported by novel biomakers predicting likely responders in secondary indications to support downstream clinical development and product launch.
Label expansion
Our platform identifies leading label expansion opportunities for additional indications and patient subgroups to increase the market potential of drug products, extend their patent life, and diversify their revenue streams.
We also support future development with mechanism-based biomarkers that can be reduced to practice as inclusion criteria for the design of efficacy trials and as low-cost biomarker tests to identify responders to treatment.
These biomarker-driven insights make clinical trials for label expansions faster to readout and more likely to succeed.
In-licensing
We evaluate in-licensing opportunities to meet target product profiles, clinical need, and commercial goals.
Our analysis confirms an asset’s genetic linkage with disease, and its mechanism’s causality and prevalence in patients, providing unique insight into addressable market and value.
PrecisionLife biomarkers optimize, de-risk, and accelerate the asset’s clinical development and support in-market prescription as a complementary diagnostic.
Out-licensing
We identify opportunities within a portfolio to add value to programs which can be out-licensed.
Our out-licensing data packages combine primary indication data and regulatory submissions with detailed secondary indication and genetic prevalence data. This is enhanced with our biomarkers for the design of targeted clinical trials.
These biomarkers add to the IP package – enabling a precision medicine premium and making the program more attractive to potential licensing partners.
OPTIMIZE YOUR PORTFOLIO
Getting started is simple
All we need are the names and indications of the targets to be considered.
From this we can advise on how you could optimize your assets and drug portfolio, rapidly and with minimal risk.



