Overview
Neurodegenerative diseases such as Alzheimer’s disease (AD) are multifactorial, with multiple genetic and environmental components. Knowledge of the most obvious genetic correlations and pathophysiological changes observed in patients has not yet led to the development of any effective medications.
Over 100 clinical trials for drugs targeting the development of amyloid-β plaques and hyperphosphorylated tau proteins have failed. This is not because the drugs didn't impact their targets as intended, but because those targets weren't driving disease in sufficient patients in the trial to demonstrate the necessary level of clinical impact.
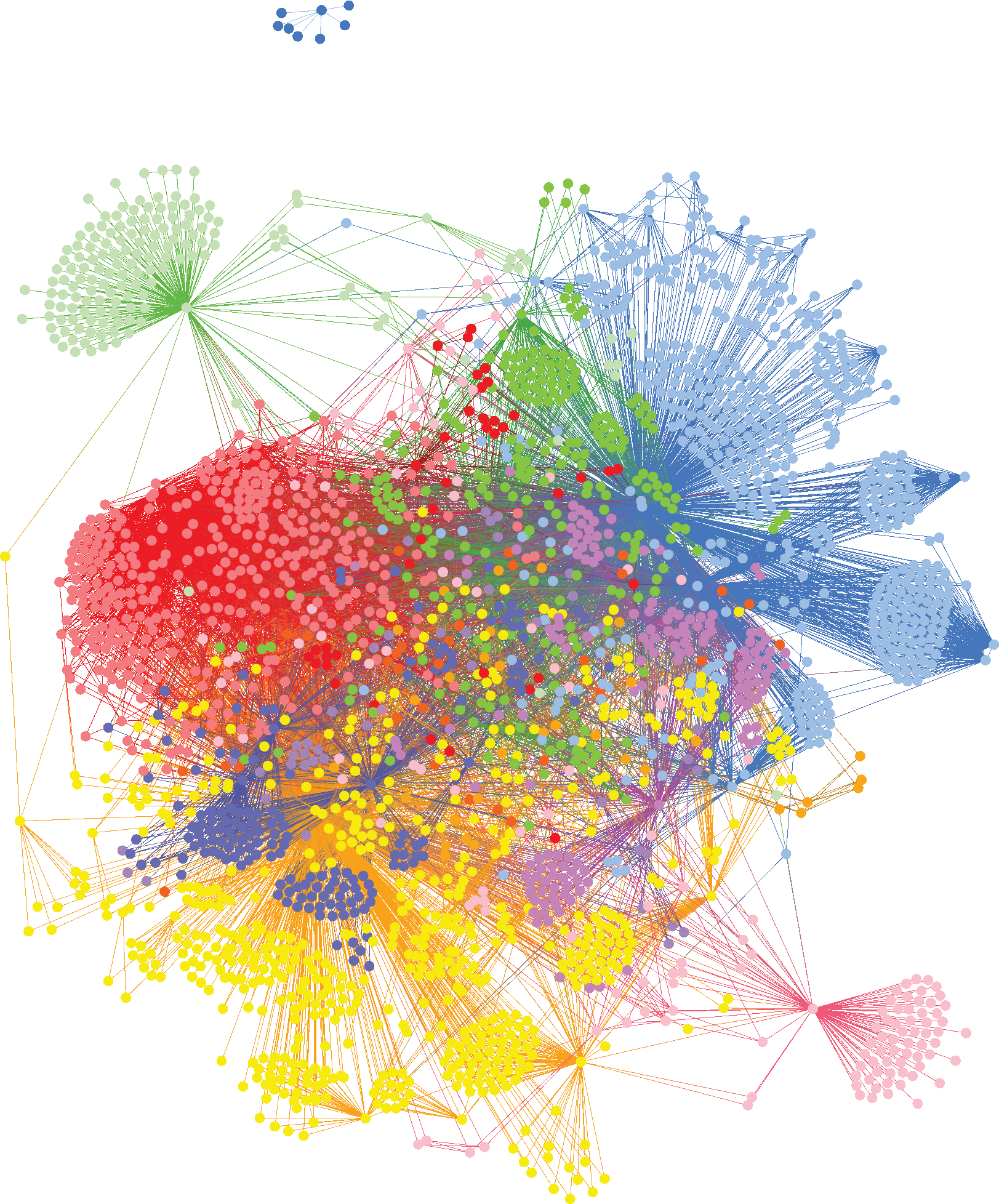
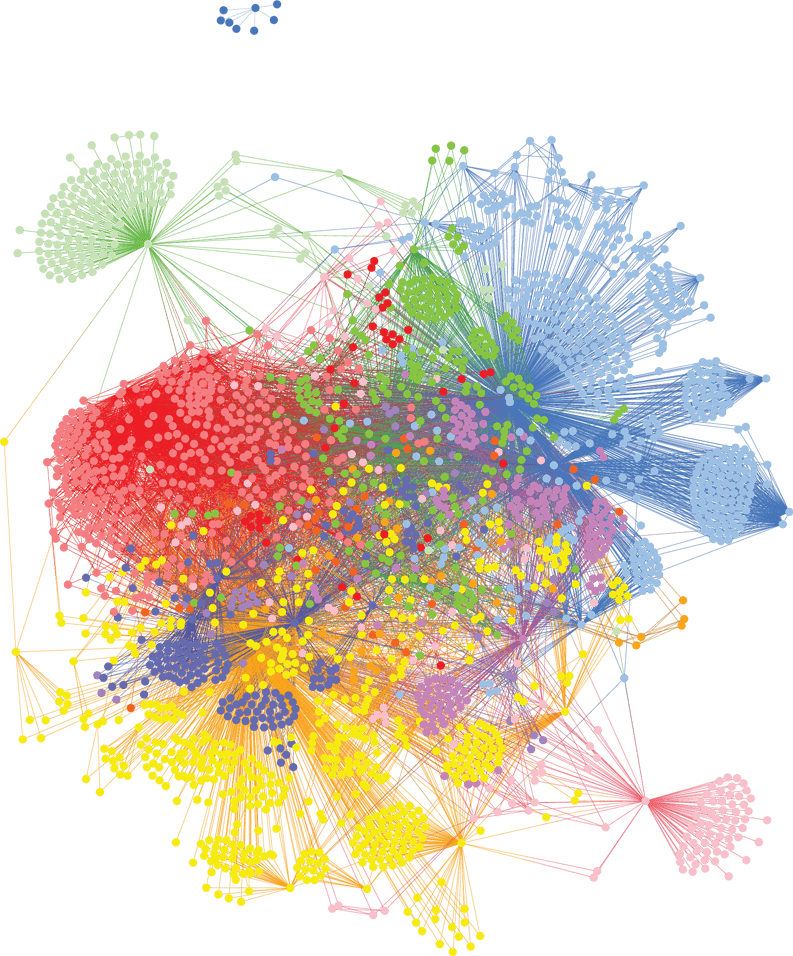
We analyzed genomic data from 882 patients diagnosed with AD against healthy controls with no family history of the disease, identifying 113 genes that are highly associated with the risk of developing AD. GWAS studies only identified the Apoε4 locus from this same dataset.
By clustering people who share the same disease-associated SNPs, we identified six major subgroups of patients with distinct AD risk genes. While highly plausible mechanistically, many of these represent novel targets for AD research. Each of these subgroups shares combinations of genes that are involved in different biological mechanisms and will benefit from a different type of medication, specific to their disease driver.
In understanding the biological mechanisms of these subgroups we can identify patients who will respond favorably to new drug candidates, to improve demonstration of efficacy and bring more effective medicines to market for people to receive personalized treatments.